VISIT US ON CPhI China, Shanghai 19-21 June 2024 / CPhI Worldwide, Milan 8-10 October 2024
THE CLOSED LOOP PRODUCTION
The EU-GMP certifies the production process conformity starting with the collection of Mucosa.
However, we are going one step further.
Already in the slaughterhouse we take everything in our own hands: Our trained staff are responsible for separating the intestines, for the transport with company-owned trucks, the incoming goods inspection and the diligent documentation of every single batch. Only this way we can ensure the closed-loop production process.


Of course, the health condition of every single animal is inspected by veterinaries before and after slaughter.
What is taken as raw material from the slaughterhouse, only leaves our company as a finished product (API). Subject to full and complete controls, documentation and EU-GMP certification.

QUALITY IS NOT A COINCIDENCE. IT IS THE RESULT OF DETAILED PLANNING, WISE FORESIGHT AND METICULOUS INSPECTION.
The entire production process is subject to full and complete inspections, is documented and EU-GMP certified.
In all stages of production we continuously take samples in order to ensure the consistent high standard. A team of specially trained and qualified staff accompanies the production process and analyzes and documents every single batch of each stage of production.

CERTIFICATION, ANALYSIS, REGULATORY
WE CONTINUALLY DEVELOP OUR PRODUCTION RANGE, OFTEN IN COOPERATION WITH OUR CUSTOMERS. PLEASE CONTACT US. WE ARE HAPPY TO ASSIST YOU!
HEPARIN RANGE
Heparin Sodium (EU-GMP)
Heparin Calcium (EU-GMP)
Enoxaparin Sodium (EU-GMP)
Dalteparin Sodium (EU-GMP)
Nadroparin Calcium (EU-GMP)
Dermatan Sulfate
Heparinoid Sodium
Mucopolysaccharide Polysulfate
Further Heparin derivatives under development
OTHER BIOLOGICAL ACTIVE SUBSTANCES
Lung Surfactant
IN ORDER TO GET WHERE WE ARE NOW HAS CERTAINLY BEEN A LONG JOURNEY. AND THAT IS JUST THE BEGINNING.

When starting production in 2015, we could already look back to 30 years of experience with Heparin.
We knew exactly the market’s requirements, where the emphasis is to lay and where we are heading.
Based on this knowledge and experience we have compiled, planned and stringently implemented our concept. No habits, no traditions nor any old structures were in our way to establish a production in Chongqing in response to the challenges of time.


Having already been awarded the EU-GMP certification for Heparin Sodium after a period of 12 months only is ample proof that this was the right approach. And we went further. Our newest achievements: EU GMP Certifications for Enoxaparin Sodium, Dalteparin Sodium and Nadroparin Calcium.
The journey continuous: Construction for our additional
site at Kaizhou, Chongqing has started.
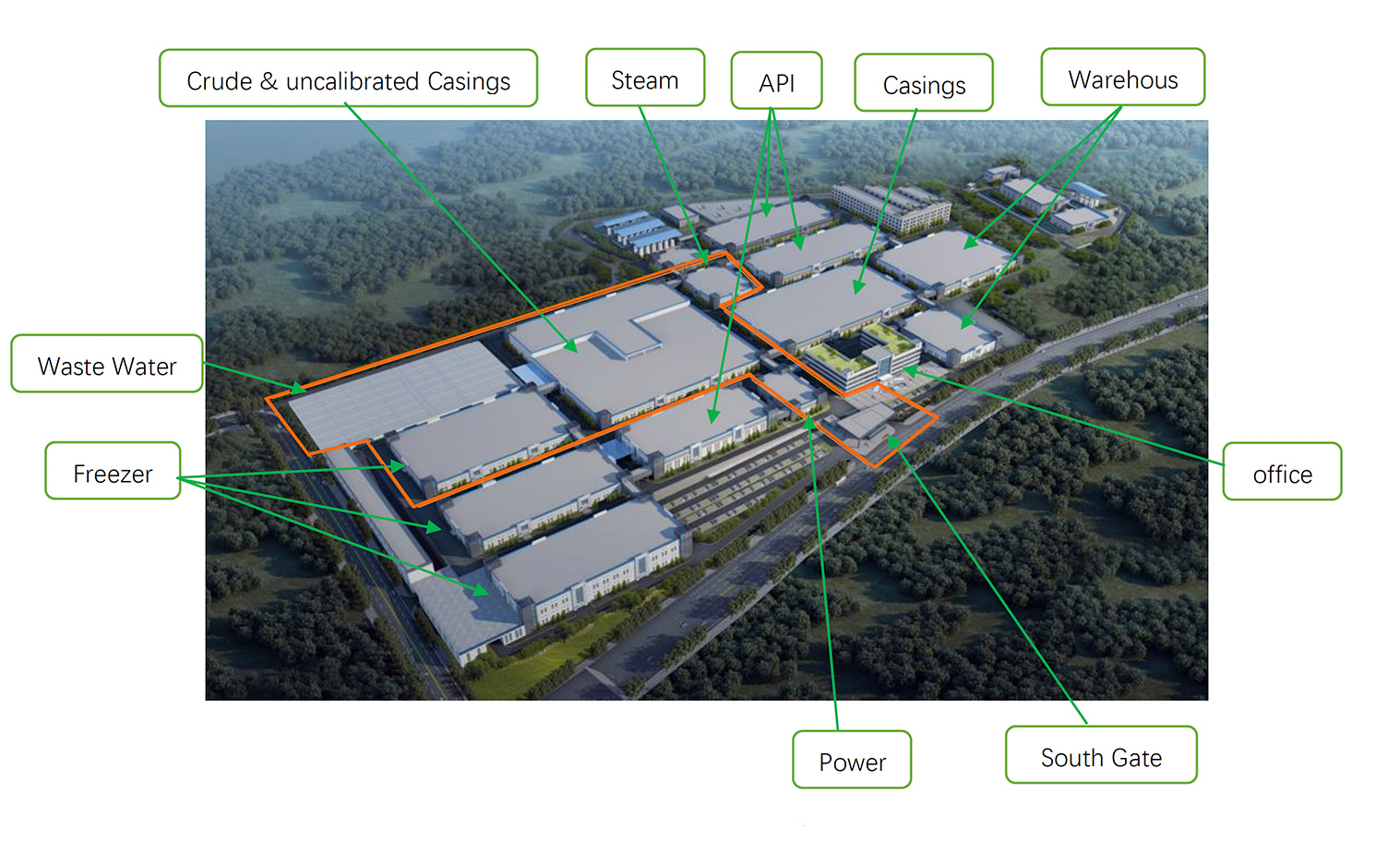
Area in orange is under construction.
Processing capacity: 6 million MIU Heparin crude per year.
Plenty of space to extend intestine processing and crude Heparin extraction. This to complement our growth and extension to range of Low Molecular Weight Heparins. We strictly follow our vision for traceability and closed loop production process.

We deliver door to door
Our branch office in Hamburg, Germany is licenced to import, store and deliver our products to customers in the EU.

WE ARE LOOKING FORWARD TO MEETING YOU: WELCOME TO CHONGQING!
Contact
Yino Pharma Limited
2 Cuiping Erxiang, Yubei District, Chongqing 401120, China
Phone +86-23-67377173 ext 8002/ 8003
info@yinopharma.com
www.yinopharma-heparin.com
Yino Pharma Europe GmbH
Hammer Deich 156, 20537 Hamburg, Germany
Phone +49-162-8790602
Imprint
Information pursuant to § 5 TMG
Yino Pharma Europe GmbH
Hammer Deich 156
20537 Hamburg
Commercial Register: HRB 144835
Registration court: Amtsgericht Hamburg
Represented by:
Matthias Kattein, Richard Yin
Contact
Phone: +491628790602
E-mail: info@yinopharma.com
VAT ID
Sales tax identification number according to § 27 a of the Sales Tax Law:
DE310299497
Supervisory authority
Behörde für Gesundheit und Verbraucherschutz der Freien und Hansestadt Hamburg, Fachabteilung V4, Pharmaziewesen und Medizinprodukte
Billstrasse 80, 20539 Hamburg
We are not willing or obliged to participate in dispute resolution proceedings before a consumer arbitration board.
Photos: Jiang Nan
Concept and design:
www.janzen-werbung.com
Web development and hosting:
www.3plus.solutions





